Challenge
Despite new medicines, successfully treating TB remains complex and challenging. Patients need a correct diagnosis and must complete a lengthy course of multiple drugs over six months. This creates heavy demands on patients, families, and health systems, especially in low- and middle-income countries with the highest TB burden.
Treatment is complicated by growing resistance to commonly used TB drugs. This occurs when:
- Patients are mismanaged medically or not well supported through the course of treatment.
- Drug supplies are unstable, unreliable or of poor quality. Some patients need further testing to confirm drug resistance and require even longer treatment with second-line drugs, which can cause significant side effects and need clinical monitoring.
- Diagnostic capacity to identify underlying resistance is not available.
Our Work
To hasten the decline of the global TB burden, reduce deaths, and allow health care systems in low- and middle-income countries to deliver more efficient, shorter and simpler TB treatment with greater success, potent novel “pan-TB” regimens to treat all patients are urgently needed.
Since its inception, the PAN-TB Collaboration has been working closely with partners to accelerate the identification of promising pan-TB regimens for advancement through phase 2b/2c clinical trials. These pan-TB regimens will be designed to have improved safety and tolerability, a shorter duration, and simpler to use than existing treatment options.
PAN-TB also conducts non-clinical studies like the relapsing mouse model studies (RMM), which are important in evaluating TB drug regimens. The RMM 2022 study has been approved and will be initiated at Evotec with the 7 priority PAN-TB compounds and drugs to evaluate the time to cure for 12 priority TB regimens. The RMM 2022 builds on learnings from earlier PAN-TB RMM studies as well as Evotec pilot studies.
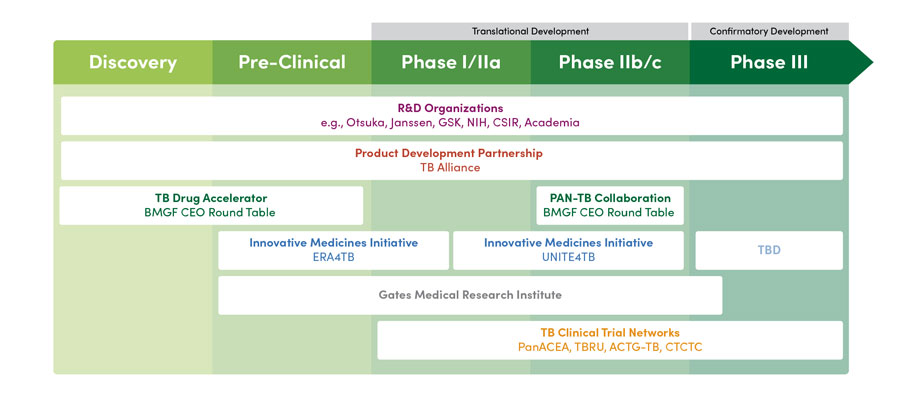
TB Research & Development Landscape

FAQs
What’s unique about this collaboration and why do we need it?
- TB treatment regimens require a combination of drugs. No single organization has a complete combination of drugs in their portfolio to develop a full regimen capable of treating all forms of TB.
- At the same time, novel drug candidates that have shown potential to treat TB have been discovered and are undergoing early pre-clinical and clinical assessment.
- The PAN-TB collaboration aims to accelerate the identification of promising pan-TB regimens and conduct research through phase 2b/2c clinical trials. The regimens will be designed to have little to no drug resistance and an acceptable safety profile, and be better-tolerated, shorter in duration and simpler to use than existing options.
Why does the PAN-TB Collaboration focus on “pan-TB” regimens instead of treatments for drug-resistant TB?
- Current tools to diagnose and treat TB are insufficient. It is difficult for providers to accurately diagnose the disease and patients must take treatments that have significant side effects for multiple months.
- Successful treatment of TB currently requires health systems and providers to diagnose the correct disease form: drug-sensitive or drug-resistant (DR-TB). And within DR-TB, different levels of resistance can be detected (e.g., MDR-TB, XDR-TB) which require varying regimens and approaches to tackle. Training and technology that enable accurate diagnosis are expensive and often lacking in areas with high TB burdens.
- Existing treatment regimens are complex and difficult for both patients and health systems. Even the least complicated forms of TB require patients to take multiple pills a day for six or more months, often with clinical monitoring required. Patients with DR-TB – which does not respond to the most commonly used medicines – face even longer and more complex treatment regimens.
- Pan-TB regimens with acceptable safety profiles that are better-tolerated, shorter in duration, and simpler to use than existing options have the potential to greatly improve outcomes for all TB patients.
- PAN-TB is evaluating five antimicrobial agents under joint development agreement, and the organizations contributing them, include:
- Bedaquiline; registered product for multidrug-resistant TB, Janssen Pharmaceutica NV, part of the Janssen Pharmaceutical Companies of Johnson & Johnson, and NCE for drug-sensitive TB, TB Alliance
- Delamanid; registered product, Otsuka Pharmaceutical Co., Ltd.
- Pretomanid; registered product, TB Alliance
- Quabodepistat; NCE, Otsuka Pharmaceutical Co., Ltd.
- Sutezolid; NCE, TB Alliance, Medicines Patent Pool, Gates Medical Research Institute
- The two investigational drug regimen combinations to be evaluated include:
- DBOS – delamanid, bedaquiline, quabodepistat and sutezolid
- PBOS – pretomanid, bedaquiline, quabodepistat and sutezolid
- PAN-TB is evaluating five antimicrobial agents under joint development agreement, and the organizations contributing them, include:
- See current studies here.
How is this initiative different from other collaborations?
- The PAN-TB collaboration is the first philanthropic, non-profit and private sector collaboration that aims to accelerate the development of treatment regimens capable of treating all forms of tuberculosis.
- The collaboration will leverage members’ collective assets, technology, resources and scientific expertise to identify and evaluate investigational drug regimens that can treat all forms of TB, have an acceptable safety profile, and be better-tolerated, shorter in duration and simpler to use than existing options.
- Other collaborations like:
- The TB Drug Accelerator (TBDA) is a collaboration of academic and industry partners that aims to speed up the discovery and development of novel preclinical drug candidates against TB by working together on early-stage pre-clinical research.
- ERA4TB plans to study new molecular entities in combination with one another and will focus on preclinical/first-in-human trials, while the PAN-TB collaboration will study new combinations of novel and existing drugs (which have already been tested in humans) in phase 2 research. If ERA4TB advances candidates that show promise in initial human studies, they could be incorporated into the PAN-TB collaboration’s later-stage research. The PAN-TB collaboration plans to work closely and coordinate transparently with the IMI/ERA4TB project to avoid duplication of efforts and harmonize approaches to the greatest possible degree to enable pooling of clinical data sets.
- UNITE4TB follows an overarching racetrack concept to deliver an efficient, global clinical trials network equipped to implement phase 2 trials that conform to the highest regulatory standards.

